Advantages
- First-in-Class Mechanism: Targets a novel pathway—protein farnesylation—for heart failure treatment.
- Broad Therapeutic Potential: Effective in both HFrEF and HFpEF, addressing a major unmet need.
- Tested in multiple preclinical models: Danon disease cardiomyopathy (genetic model); HFpEF (diet-induced and hypertensive models); HFrEF (pressure overload model).
- Translational Readiness: Builds on FDA-approved FTase inhibitors (e.g., Lonafarnib) with known safety profiles.
Summary
Despite advances in pharmacological treatments for heart failure with reduced ejection fraction (HFrEF), there are limited effective therapies for heart failure with preserved ejection fraction (HFpEF), which account for more than half of all HF cases. Mortality rates remain high, with approximately 50% of patients dying within five years of diagnosis. Current therapies fail to address the underlying molecular mechanisms of HFpEF and cardiomyopathy, particularly protein aggregation and mitochondrial dysfunction in cardiomyocytes.
This invention introduces a novel therapeutic approach targeting farnesyltransferase (FTase)—an enzyme involved in post-translational modification of proteins— to restore proteostasis and cardiac function.
Using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) from patients with Danon disease, researchers conducted high-throughput screening and identified 5 small molecules FTase inhibitors that significantly improved mitochondrial function and reduced protein aggregation. Notably, Lonafarnib and Tipifarnib are FDA-approved drugs. The lead compound is a highly potent small molecule with an IC₅₀ of approximately 0.5 nanomolar. In mouse models of heart failure with preserved ejection fraction (HFpEF), FTase inhibition reversed cardiac hypertrophy, reduced fibrosis, improved exercise capacity, and prevented mortality. This invention offers a transformative approach to heart failure therapy by targeting the molecular root cause—protein aggregation driven by FTase activity.
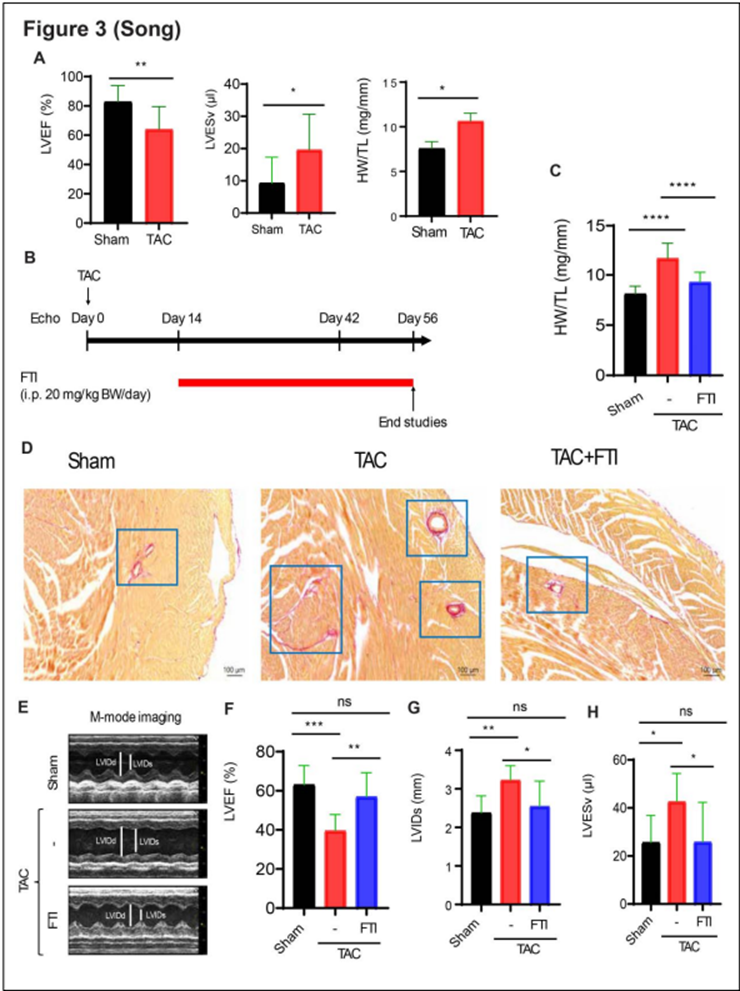
FTI227, a potent farnesyltransferase inhibitor, reverses cardiac remodeling and restores systolic function in a mouse model of HFrEF: A) Mice subjected to TAC showed a 23% decrease in left ventricular ejection function (LVEF), a 100% increase in left ventricular end-systolic volume (LVESv), and a 44% increase in the ratio of heart weight to tibia length (HW/TL). B) Experimental design. C) FTI treatment reversed cardiac hypertrophy, shown as ratio of heart weight to tibia length (HW/TL). D) Fibrosis assessment (PicroSirius Red staining) of heart sections. FTI treatment reversed cardiac remodeling in HFrEF mice. E-H) Echocardiography measurement of systolic function of left ventricle after 8 weeks of TAC.
Desired Partnerships
- License
- Sponsored Research
- Co-Development