Advantages
- Precise spatiotemporal control and reversible modulation of kinase activity
- Reduced systemic toxicity and off-target effects
- Superior binding affinity and isomer selectivity than traditional Jak 2 inhibitor
Summary
Janus kinase 2 (JAK2), a key player in hematologic cancers and autoimmune diseases, is a particularly challenging target due to its structural similarity with other kinases. While traditional small-molecule kinase inhibitors have revolutionized cancer therapy, they suffer from poor specificity, selectivity, and the development of drug resistance. These inhibitors lack the ability to precisely control drug activity in space and time, leading to off-target effects and limited therapeutic windows. Photopharmacology—a cutting-edge field combining pharmacology and photochemistry—offers a promising solution by enabling light-controlled activation and deactivation of drugs at specific sites.
Our researchers have developed novel photoswitchable small-molecule inhibitors specifically designed for JAK2. Through extensive computational modeling, including molecular docking and simulations, IPTA (Isopropyl, Phenyl, Trifluoro, and Amide/amine group) was engineered. The molecule incorporates a light-responsive azobenzene core that toggles between two isomers: the active E-isomer, which binds selectively to JAK2, and the inactive Z-isomer, which disrupts binding through steric clashes. This reversible switching mechanism allows for precise spatiotemporal control over drug activity, minimizing off-target effects, enhancing target selectivity, and expanding the therapeutic window to improve efficacy and safety.
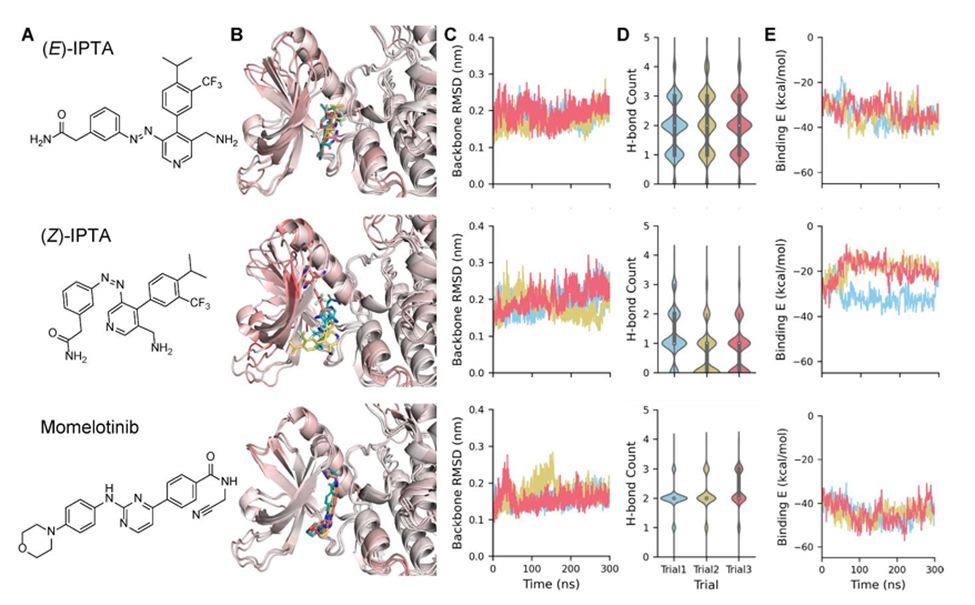
Comparative Dynamics Simulation of IPTA Isomers and FDA-Approved JAK2 Inhibitors: A) Chemical structures. B) Representative cluster conformations superimposed and colored by RMSD (darker red indicates greater deviation), highlighting the consistent binding of (E)-IPTA and momelotinib, in contrast to (Z)-IPTA. C) Kinase backbone Root Mean Square Deviation (RMSD) fluctuations. D) Distribution of hydrogen bond counts throughout the simulations (E-IPTA forms persistent hydrogen bonds comparable to FDA-approved inhibitors). E) Binding free energy profiles of the complexes over time (E-IPTA has strong and stable binding affinity similar to approved drugs).
Desired Partnerships
- License
- Sponsored Research
- Co-Development