Adantages
- Enhanced Blood-Brain Barrier (BBB) Penetration allows for efficient therapeutic targeting
- Superior serum stability enables a longer therapeutic window compared to conventional peptide therapeutics
- Multimodal therapeutic mechanism simultaneously addresses multiple pathological hallmarks of Alzheimer’s Disease (AD)
Summary
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder driven by the accumulation of amyloid-β (Aβ) peptides, which form neurotoxic oligomers and fibrils. These aggregates disrupt neuronal function, leading to synaptic loss, mitochondrial dysfunction, oxidative stress, and neuroinflammation—ultimately resulting in cognitive decline. While extensive efforts have been made to target Aβ pathology, current therapeutic strategies face limitations, including poor specificity, toxicity, low blood-brain barrier (BBB) penetration, and serum instability—particularly among peptide-based inhibitors.
Our researchers have engineered M4, a peptidomimetic α/sulfonyl-γ-AApeptide foldamer designed to selectively bind the central domain of Aβ, stabilizing its partial helical structure to block the formation of neurotoxic β-sheet aggregates and disrupt existing ones. Unlike traditional peptide inhibitors, M4 exhibits high serum and enzymatic stability, effective BBB permeability, and a multimodal mechanism of action involving the preservation of synaptic and mitochondrial integrity, reduction of neuroinflammation, and restoration of cognitive function in preclinical AD models. In vitro studies showed that M4 effectively blocked the formation of toxic amyloid-beta aggregates and disrupted existing ones, while protecting neurons by restoring mitochondrial function and reducing inflammation. In vivo experiments using Alzheimer’s mouse models demonstrated that M4 reduced plaque buildup, improved brain cell health, and significantly enhanced learning and memory.
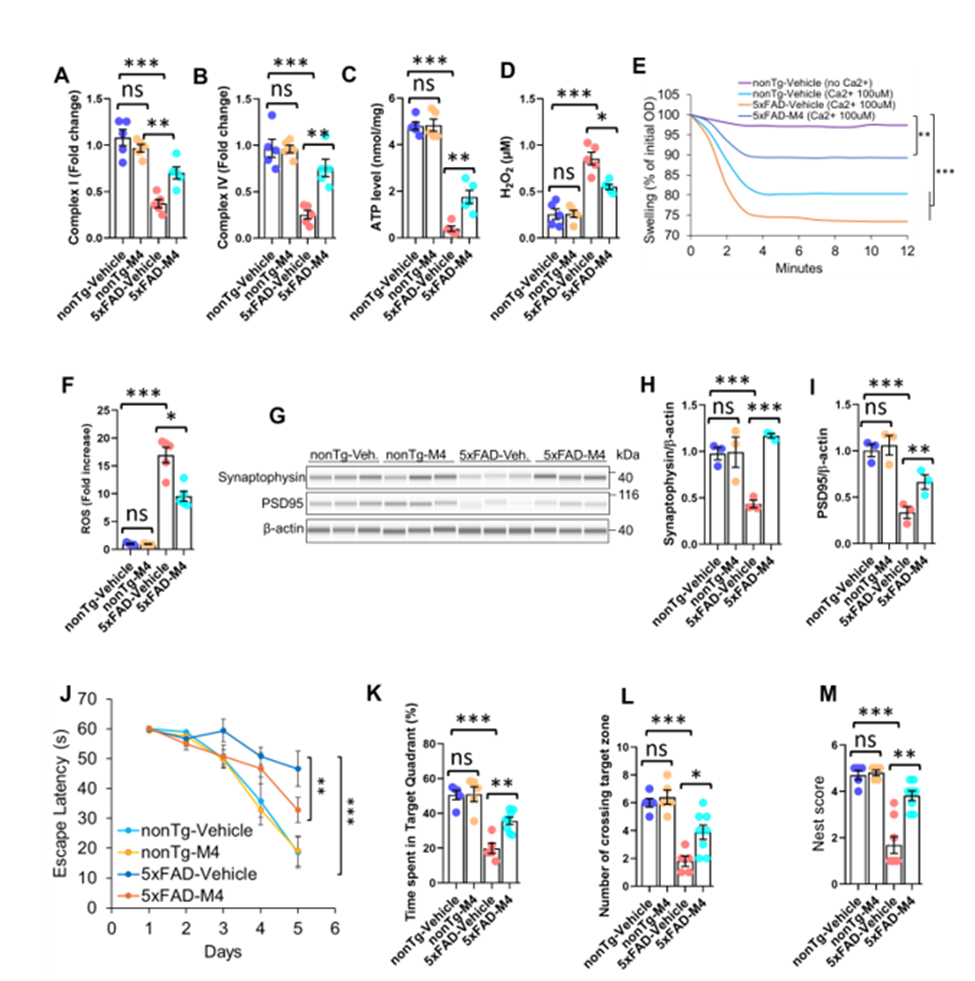
1. Subfigures J–L (MWM): Behavioral improvements are critical for demonstrating real-world therapeutic benefit.
2. Subfigures A–C (Mitochondrial restoration): Strong mechanistic evidence of disease modification.
3. Subfigures G–I (Synaptic repair): Direct link to cognitive function and neuroprotection.
Desired Partnerships
- License
- Sponsored Research
- Co-Development