Advantages
- Enhanced Immunogenicity: Nanoparticles (NPs) boost immune responses compared to conventional subunit vaccines
- Broad Protection: Focusing on conserved regions of CSP, the vaccine elicits cross-reactive antibodies that may protect against multiple P. vivax strains
- Functional Efficacy: Generated antibodies inhibit sporozoite invasion of liver cells in vitro
- Improved Stability & Safety: PLGA-based NPs are biodegradable, biocompatible, and allow for controlled antigen release, reducing the need for multiple doses
Summary
Plasmodium vivax is a protozoal parasite responsible for malaria disease. Sporozoites, the infective stage of the malarial parasite, are considered ideal targets for antimalarial strategies. The circumsporozoite protein (CSP) is the most abundant molecule on the surface of plasmodium sporozoites and is considered a leading pre-erythrocytic stage (PE) vaccine candidate. Limitations of conventional vaccines such as low immunogenicity, toxicity, instability and the need for multiple doses of conventional vaccines is a challenge for vaccine efficacy.
Our researchers developed and tested a nanoparticle-based vaccine platform that co-deliver recombinant P. vivax CSP antigens and adjuvants directly, to target lymphoid tissues and immune cells for enhanced immune stimulation. They developed biodegradable PLGA nanoparticles (NPs) conjugated with recombinant CSP antigens, including full-length CSP (CSP FL), its N-terminal (CSP NT), C-terminal (CSP CT), and a combination of both (CSP NT+CT), as well as CRR-derived peptides. The nanoparticle vaccines were tested in BALB/c mice and demonstrated high antibody titers against the respective CSP constructs. Notably, the CSP NT+CT formulation induced stronger and broader immune responses than the individual subdomains. These antibodies not only recognized native CSP on P. vivax and transgenic P. berghei sporozoites but also effectively inhibited sporozoite invasion of hepatocytes in vitro.
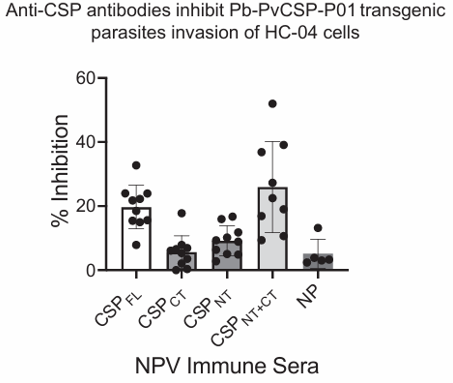
Functional activity of anti-CSP-P01 NPV immune sera. Sera from mice (n=10) immunized with CSPFL, CSPNT and CSPCT and CSPNT+CT NPVs were evaluated for inhibition of transgenic Pb-PvCSP-P01 sporozoites infectivity of hepatocytes in vitro by ILSDA. Each data point on the graph represents individual mouse serum tested at 1:100 dilution and error bars represent means and standard deviations.
Desired Partnerships
- License
- Co-Development
- Sponsored Research