Advantages
- Provides a preventative approach to improving vision and reducing post-procedure complications
- Does not compromise or change donor tissue quality or viability
- Can be accomplished with a variety of techniques available in the market
- Drug dose and release can be controlled to meet patient specific needs
Summary
Partial and full thickness keratoplasties are clinical procedures that involve replacing part or the entirety of the cornea with donor corneal tissues. Post-transplant complications such as inflammation, infection, corneal rejection, and glaucoma can occur. Presently, there are no preventative treatments for these complications and if they arise, they are treated after the transplant. If sub optimally treated, these complications may potentially cause eye damage.
Our inventors developed a method of impregnating donor corneal tissues with agents that prevent tissue rejection prior to transplantation. The agents can range from anti-infectives, antiglaucoma therapy, corticosteroids, anti-inflammatory medications and more. This presents a novel preventative approach to complications that may arise after corneal transplantation, which are commonly treated with medicated drops. Pre-loading the corneal tissues with drugs reduce the overall risk of infection, graft rejection, glaucoma, and uveitis.
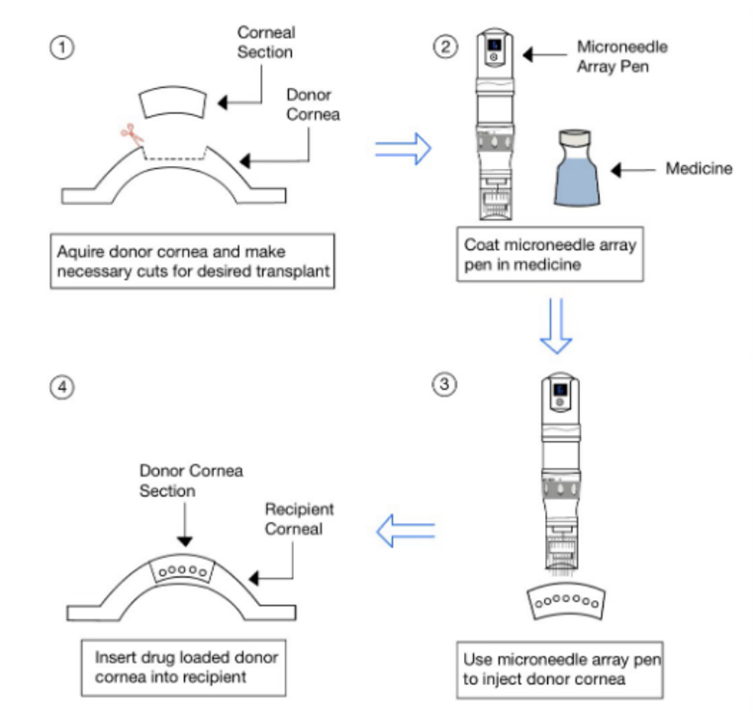
Diagram of stepwise drug loading into corneal donor tissue using microarray needle and transplant into recipient.
Desired Partnerships
- License
- Sponsored Research