Advantages:
- Discovered novel structural interactions between HS and TREM2, associated with neurodegeneration
- High-affinity binding: TREM2 specifically interacts with Heparan Sulfate (HS) modified with 6-O-sulfation and iduronic acid, enabling precise microglial modulation compared to broad-spectrum anti-inflammatory strategies
- Defined structural requirements: HS chains require a minimum of 10 saccharide units with critical 6-O-sulfation for effective TREM2 binding and ApoE3 uptake, providing clear parameters for therapeutic design
- Precision therapy potential: Enables the development of targeted neuroimmunomodulatory therapies for Alzheimer’s disease (AD) and other neurodegenerative disorders by enhancing TREM2-mediated clearance of amyloid-beta lipoproteins, apolipoproteins, phospholipids, and sphingolipids
Summary:
Neurodegenerative diseases, including Alzheimer’s disease (AD), are exacerbated by microglial dysfunction, driven partly by impaired TREM2 signaling. TREM2, a microglial receptor, is critical for phagocytosis, lipid metabolism, and immune modulation under physiological conditions, but its dysfunction—linked to variants like R47H and R62H—accelerates AD pathology by impairing amyloid-beta clearance and promoting neuroinflammation . TREM2 interacts with Heparan Sulfate (HS) as a modulator of microglial function (); however, limited knowledge about the specific binding interactions between TREM2 and HS hinders the ability to design interventions that restore TREM2-mediated neuroprotection in disease states.
Our inventors identified microglial heparan sulfate (HS) as a key regulator of amyloid-β (Aβ) clearance in Alzheimer’s disease (AD). The study reveals that HS suppresses the function and expression of Triggering Receptor Expressed on Myeloid cells 2 (TREM2), a receptor critical for microglial Aβ phagocytosis. By forming HS-TREM2 complexes, HS inhibits TREM2’s ability to bind Aβ, thereby impeding its clearance and contributing to AD pathology. Knockout experiments targeting HS biosynthesis genes (Ndst1 and Hs6st1) in microglia demonstrated enhanced Aβ clearance, increased beneficial disease-associated microglia (DAM), and improved cognitive function in AD mouse models. This research introduces the concept of selectively reducing microglial HS—particularly its 6-O-sulfation—as a promising therapeutic strategy for AD, with significant implications for the pharmaceutical industry in developing targeted treatments that modulate microglial activity and neuroinflammation.
Microglial HS Reduction Reverses Alzheimer’s Pathology and Restores Cognitive Function
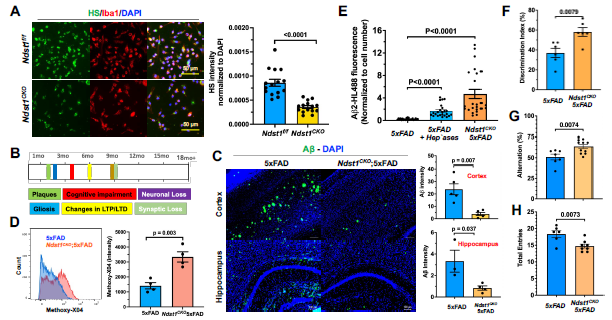
Targeted knockout of the HS biosynthesis gene Ndst1 in microglia significantly reduces amyloid-β (Aβ) plaque deposition, enhances microglial Aβ phagocytosis, and improves memory performance in 5xFAD Alzheimer’s mouse models. Immunostaining and flow cytometry reveal increased uptake of Aβ fibrils, while behavioral tests show restored object recognition and spatial memory. These findings highlight microglial HS as a novel and actionable therapeutic target for halting AD progression and rejuvenating brain function.
Desired Partnerships:
- Sponsored Research
- Co-Development