Advantages:
- A novel structural diversity (anthoteibinenes A-Q) offers bioactive scaffolds, absent in standard combinatorial chemistry libraries
- Anthoteibinenes I and J have demonstrated activity against multiple Candida strains, indicating possible antibacterial efficacy
- Selective bioassay- and NMR-guided isolation provides a more targeted approach improving efficiency and reducing costs
- Enhanced chemical space for drug discovery offering fresh chemical backbones not easily found in existing libraries
Summary:
Deep-sea organisms are increasingly recognized as sources of novel bioactive substances, yet unlocking their therapeutic potential presents substantial challenges in both discovery and development. Isolation efforts often face sampling difficulties, low compound yields, and complex structural features rarely encountered in terrestrial metabolites. Traditional fractionation procedures can be inefficient for pinpointing trace constituents with meaningful activity, while elucidating intricate structural motifs—including nitrogen- and halogen-bearing functionalities—frequently requires a combination of advanced spectroscopic methods, high-resolution mass analyses, and computational chemistry techniques. Moreover, the narrow or selective bioactivity often observed in newly identified marine-derived compounds underscores the challenge of discovering broad-spectrum agents and highlights the need for targeted screening, rigorous structure-activity investigations, and alternative drug development strategies to combat rising antimicrobial resistance in clinical settings.
Our researchers studied the chemodiversity of the deep-sea soft coral Anthothela grandiflora and report 17 new cadinene-like functionalized sesquiterpenes, anthoteibinenes A-Q. Four of the terpenoids feature an unprecedented dimethylamine moiety with one or two carbon linkers, and one anthoteibinene is halogenated. Their isolation was guided by both bioassays and ¹H NMR signals, focusing on isopropyl methyl resonances, with final structures confirmed through an advanced combination of NMR (1D/2D), high-resolution mass spectrometry, DFT-based NMR calculations (DP4+), and electronic circular dichroism. Unlike previously reported cadinene, these uniquely substituted analogues exhibit selective antifungal activity associated with specific phenolic groups, while showing no activity against ESKAPE pathogens or in anti-inflammatory assays.
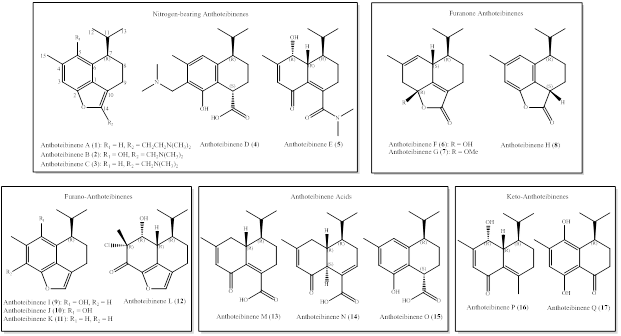
Figure 1. Anthoteibinene A-Q, sesquiterpenes from the Irish deep-sea coral Anthothela Grandiflora.
Desired Partnerships:
- License
- Sponsored Research
- Co-Development