Advantages:
- 15dPGJ2 blocks the proliferation of human endometrial endothelial cells, thereby preventing pLARC-induced aberrant endometrial angiogenesis, a major cause of unexpected uterine bleeding
- 15dPGJ2 enhances progestin responsiveness and prevents pLARC-induced inflammation, another contributor to uterine vascular instability, effectively reducing pLARC-induced AUB
- Since AUB is the most common cause of discontinuing pLARC therapy within the first six months of usage, 15dPGJ2 will improve contraceptive adherence, thus, offering significant financial advantages over standard pLARC
- Compatible with widely used contraceptive devices
Summary:
Progestin-only long-acting reversible contraceptives are highly effective for birth control but often lead to abnormal uterine bleeding, the primary reason for early discontinuation. This bleeding arises from reduced endometrial perfusion. Leading to hypoxia/reperfusion injury, which in turn trigger oxidative stress and subsequent inflammation. As a result, fragile, thin-walled blood vessels form, lacking proper smooth muscle support and prone to rupture. Moreover, the heightened expression of a key co-chaperone protein, FKBP51, interferes with progesterone and glucocorticoid receptors function, further exacerbating inflammation and undermining the normally protective effects of progestins. These intertwined factors collectively pose a significant challenge to maintaining consistent contraceptive use and motivate ongoing research to address the underlying mechanisms of bleeding.
Our researcher's invention targets a key molecular mechanism behind abnormal uterine bleeding (AUB) caused by progestin, only long-acting reversible contraceptives (pLARCs): elevated FKBP51 expression, which impairs progesterone and glucocorticoid receptor function, disrupts normal vascular stability, and promotes inflammation. By introducing 15-deoxy-Δ12, 14-prostaglandin J2 (15dPGJ2), the approach uniquely restores progestin responsiveness through inhibition of pLARCs-induced FKBP51, reduces inflammatory mediators, and restrains endometrial endothelial cell proliferation. Unlike conventional treatments that fail to address FKBP51’s role in driving endometrial fragility and AUB, this solution leverages 15dPGJ2 to preserve the anti-inflammatory properties of progestins and stabilize the endometrial vasculature, offering a novel strategy to reduce the high discontinuation rates associated with pLARCs.
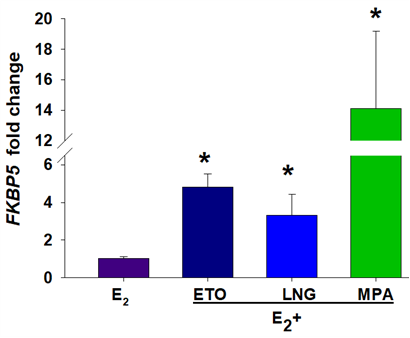
Figure 1. pLARCs treatment increases FKBP5 mRNA in cultured human endometrial stromal cells (HESCs) treated with 10−8 M estradiol (E2) ± 10−7 M etonogestrel-ETO, levonorgestrel-LNG or medroxyprogesterone acetate, MPA for 7 days. Compared to E2 alone, progestins (ETO, LNG, or MPA) increased FKBP5 expression. *P<0.05 vs. E2
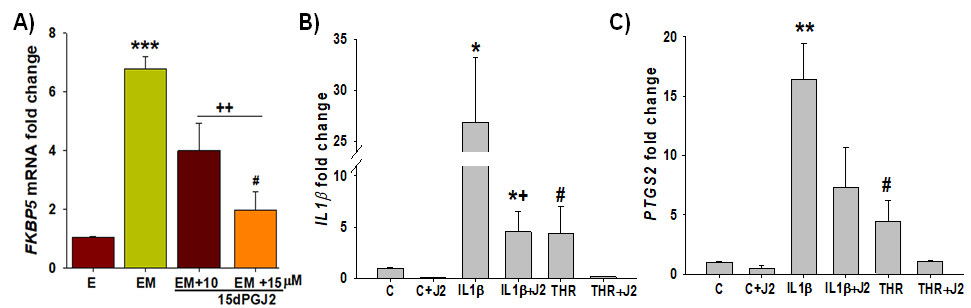
Figure 2. 15dPGJ2 inhibits FKBP51 and displays anti-inflammatory effects in HESCs. A) 15dPGJ2 significantly inhibited MPA-induced FKBP5 levels in a dose-dependent manner. B & C) both IL-1β and thrombin significantly increased expression of IL1B by ~26- and 4-fold, respectively and PTGS2 (COX2) by ~16- and 4-fold, respectively vs. control; and 15dPGJ2 significantly reversed IL-1β or thrombin-induced IL1B and PTGS2 levels
Desired Partnerships:
- License
- Sponsored Research
- Co-Development