Advantages:
- Novel cyclic γ-AA peptide (M-2-5) effectively binds with EGFR and inhibits tumor growth in an A549 lung cancer xenograft model.
- M-2-5 interferes with EGFR-EGF binding, prevents EGFR phosphorylation, and suppresses cancer cell proliferation and migration.
- Targets the extracellular domain of EGFR with high affinity and specificity.
- Small molecular weight, high stability, and selective for cancer cells.
Summary:
Epidermal growth factor receptor (EGFR) is an extracellular protein ligand-receptor targeted in the development of novel and selective anticancer therapies due to its ability to control key cellular transduction pathways and regulate the growth and differentiation of many cell types. EGFR has an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. EGFR inhibitor drugs targeting the intracellular catalytic domain lack specificity and exhibit severe toxic side effects. EGFR inhibitor drugs targeting the extracellular domain have already been successfully developed for clinical therapeutic strategies. Despite the success of EGFR-targeted drugs, there is a high demand for EGFR therapeutics with lower costs and improved efficacy.
Our researchers have discovered a novel cyclic γ-AA peptide (M-2-5), that can interfere with EGFR-EGF binding and inhibit EGFR phosphorylation and downstream signal transduction. M-2-5 can also ensure the suppression of cell proliferation and restrict cellular migration. It is completely resistant to proteolytic degradation and has demonstrated robust antitumor activity in tumor xenografts. M-2-5 targets the extracellular domain of EGFR and does not require good cell permeability. Additionally, M-2-5 shows excellent selectivity between cancer cells and normal cells. The ability of M-2-5 to mimic monoclonal antibodies, combined with its small molecular weight and high stability, makes it a promising candidate for the development of novel anti-cancer therapeutics.
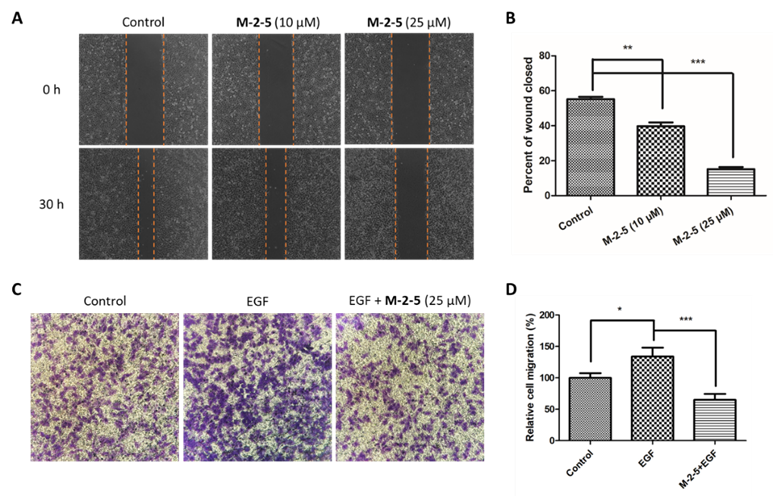
M-2-5 inhibits cell migration in vitro. (A) Wound-healing assay on A549 cells in the absence or presence of M-2-5 (10 and 25 µM) at 0 and 30 h after wound scratching. (B) The percentage of wound healing covered at 30 h.. (C) Transwell assay migration assay of A549 cells upon treatment of EGF alone or EGF+ M-2-5 (25 µM). (D) Quantitative results of migration assays.
Desired Partnerships:
- License
- Sponsored Research
- Co-Development