Advantages:
- Therapeutic Applications: 2-Aminothiazole scaffold is known for its wide range of therapeutic properties, including antioxidant, anti-inflammatory, antimicrobial, and anticancer effects.
- Compounds showed increased Nampt enzymatic activity
- Efficient Synthesis: efficient one-pot synthetic route using resorcinarene cavitand glycoconjugates (RCGs) in aqueous media, which is a green and practical approach.
- Potential treatment for wound healing, heart disease, and skeletal muscle regeneration.
Summary:
The 2-Aminothiazole scaffold is significant in medicinal chemistry due to its therapeutic properties, including antioxidant, anti-inflammatory, antimicrobial, and anticancer effects. This scaffold is found in various commercial drugs used to treat conditions such as peptic ulcers, respiratory infections, and as SSRIs and NSAIDs.
USF researchers synthesized novel aniline aromatic ring-substituted 2-Aminothiazole derivatives and evaluated their bioactivity, focusing on their effects on the Nicotinamide phosphoribosyl transferase (Nampt) enzyme, which is involved in energy metabolism and implicated in aging and metabolic diseases.
The compounds were characterized using 1H- and 13C-NMR spectroscopy, mass spectrometry, and HPLC analysis. The compounds JG-49, JG-62, and KBA-18 had good binding affinities to the Nampt activation site. In vitro assays demonstrated that all three compounds increased Nampt enzymatic activity without showing cytotoxic effects on mouse C2C12 myoblasts. These new compounds showed beneficial effects in an in vitro wound healing assay. P7C3, an earlier version of Nampt activator, decreased inflammation and improved post-injury contractility in animals.
The researcher also discovered a three-component one-pot synthesis route using resorcinarene cavitand glycoconjugates (RCGs) under aqueous conditions. This synthesis route has several advantages such as high reaction yield, shorter reaction time, and mild reaction conditions.
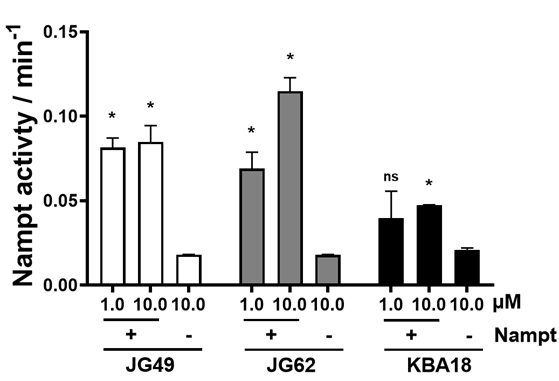
Fig. 1. Nampt enzymatic activity of the 2-Aminothiazole substituted derivatives, JG49, JG62, and KBA18 at 1.0 and 10.0 µM concentrations with (+) and without (-) the recombinant Nampt enzyme. The Nampt activity was assessed by measuring the optical density at 450nm for every 5 minutes up to 30 minutes and represented as the Nampt activity per minute. The data are represented as mean ± SEM of duplicate samples/group; where, *p<0.05.
Desired Partnerships:
- License
- Sponsored Research
- Co-Development