Advantages:
- Novel synthesis of Chondroitin sulfate (CS) particles for encapsulation of antibiotic and antifungal drugs.
- Synthesis can be fine-tuned to produced non-degradable, slightly degradable, and completely degradable CS drug carriers, for a variety of therapeutic applications.
- CS particles were successfully tested for encapsulation of tobramycin and amikacin antibiotics as well as antioxidant rosmarinic acid drug loading.
Summary:
Chondroitin sulfate (CS) is a non-immunogenic and nontoxic sulfated glycosaminoglycan (GAGs) comprised of N-acetyl galactosamine and glucuronic acid, which is naturally found in proteoglycans in connective tissues. CS possess properties such as non-toxicity, biocompatibility, and biodegradability, which make them ideal for biomaterial applications.
Our inventors synthesized CS particles in a single step with fine tuneable degrading properties (non-degradable, slightly degradable, and completely degradable) for the sustained and controlled delivery of antibacterials and antifungals drugs. During synthesis, fine tuning the mole ratio of the crosslinker, divinyl sulfone (DVS), enables the synthesis of CS particles which can be non-degradable, partially degradable, and entirely degradable particles. In one study, the non-degradable CS particles were used as a carrier biomaterial to load Tobramycin and Amikacin antibiotics via an encapsulation technique for the treatment of P. aeruginosa keratitis, which cause ulcers on the cornea. In vitro tests showed that both CS-Tobramycin particles and CS-Amikacin particles increased the inhibition zone by a disc diffusion assay. In another study, a CS microgel was utilized as an active carrier for the antioxidant rosmarinic acid (RA) with a 32.4 ± 5.1 µg/mg RA-loading capacity and sustainable and long-term RA delivery up to 150 hours.
This innovation enables the sustained delivery of antibiotics and antioxidants as an alternative to currently used systems such as drops or pills. Moreover, CS particle can used for the controlled and prolonged delivery of other drugs such as fungicide and anticancer drug due to their non-toxic and blood compatibility nature.
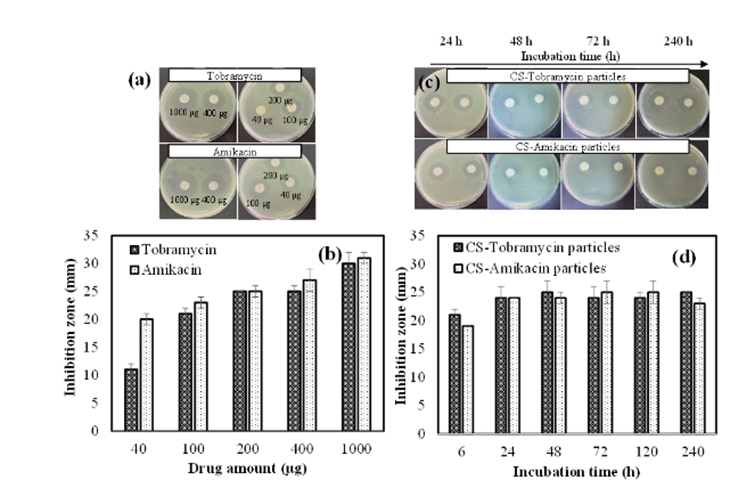
Image Description: (a, b) Inhibition zone (mm) for tobramycin and amikacin drugs at different concentrations; 20 μL of 2-50 mg/mL for 24 h incubation, and (c, d) drug-loaded CS-Tobramycin and CS-Amikacin particles at 50 μL of 50 mg/mL concentration against Pseudomonas aeruginosa ATCC 10145 at different incubation times.
Desired Partnerships:
- License
- Sponsored Research
- Co-Development