Advantages:
- The small size of the TBP (<8 kDa) allows for superior tissue penetration, making it ideal for applications requiring effective targeting of specific sites.
- Derived from a hyperthermophilic bacteria, the TBP's stability in reducing conditions and absence of disulfide bonds ensure structural integrity in challenging environments.
- With a melting temperature above 80°C, the TBP's thermal stability allows for versatile applications, including imaging agents, where it withstands extreme conditions during radiolabeling and purification.
- TBP multiple applications: Imaging Agents, Therapeutic Delivery, Biosensors
Summary:
This technology addresses the limitations of antibodies in certain applications, offering a thermophilic binding protein scaffold (TBP) derived from a hyperthermophilic bacteria, specifically the cold shock protein (CSP) from Thermatoga Maritima. TBP provides a versatile platform for developing non-antibody binding proteins with unique advantages. Its small size, stability, monomeric nature, and absence of disulfide bonds make it suitable for diverse applications, including intracellular expression, molecular imaging, therapeutic delivery, and biosensors. The TBP's distinct features, such as the ability to withstand extreme conditions and the provision of three different structural architectures for paratope development on a single scaffold, set it apart from existing technologies.
Unlike traditional antibodies and other protein scaffolds, TBP offers a combination of small size, thermotolerance, stability in reducing environments, and the capability for rational design or random library screening. The scaffold's hyperthermophilic origin and human orthologous domains enable humanization for therapeutic applications. Furthermore, the tripartite library approach, combining randomization at three distinct regions, enhances diversity. While similar in concept to other scaffolds like Affibody, TBP's unique attributes make it a standout choice for applications where antibodies may be impractical or suboptimal, such as intracellular targeting, molecular imaging, and biosensor development in challenging environments.
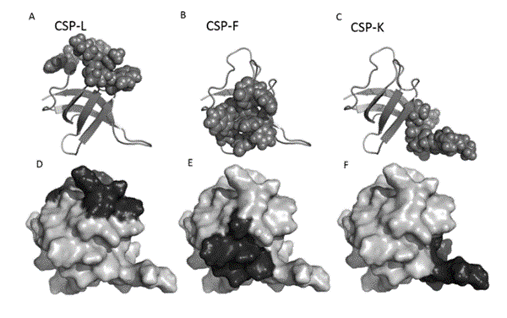
The Structural view of Thermatoga Maritima Cold Shock Protein (CSP) looks like a chain with specific parts that we want to change. Imagine three areas on this chain where we can mix up certain building blocks (amino acids). These areas are like spots on the chain's surface. Mixing up these building blocks in those spots allows us to create different protein versions. This diversity helps make proteins that can stick to specific targets, useful for various applications.
Desired Partnerships:
- License
- Sponsored Research
- Co-Development