Advantages:
- Developed lead compound #67 to elevate GAS5 levels and also enhances neuronal insulin signaling, offering a comprehensive approach to neurodegenerative complexities.
- Noninvasive Insights: GAS5's presence as a serum biomarker enables noninvasive disease assessment and NPC86 treatment efficacy confirmation.
- Compound #67's unique mechanism suggests potential synergy with existing therapies, augmenting the arsenal against neurodegenerative diseases.
Summary:
The long noncoding RNA (lncRNA) GAS5 is a regulatory lncRNA with roles in regulation of transcription, stability, and activity of receptors. USF researchers have demonstrated that GAS5 levels decrease with age in Alzheimer's disease, which is associated with increases in tau phosphorylation as well as increases in neuroinflammation, which are primary determinants of neurodegenerative diseases.
Researchers have sought to stabilize GAS5 levels in the brain. They have developed compound #67, a derivative from their first GAS5 stabilizing compound (NPC86), which shows increased efficacy to stabilize and increase GAS5 in neuronal cells. This progress is fortified by evidence establishing GAS5 as a novel therapeutic target for Alzheimer’s and related dementia. Notably, GAS5's impact on tau phosphorylation is substantial. The small molecule NP-C86, previously identified by the researchers, demonstrates the potential to increase GAS5 levels in vitro and in vivo, subsequently enhancing neuronal insulin signaling and reducing neuroinflammation. Furthermore, the presence of GAS5 in serum as a biomarker offers noninvasive insights into disease states, potentially confirming the efficacy of NPC86 in targeting GAS5.
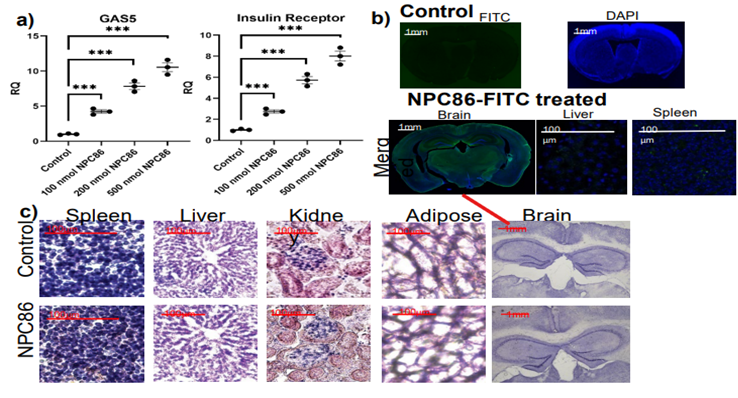
Young mice treated intranasally with increasing doses of NP-C86 for 48 hours. (a) qPCR showing dose responsive increase in GAS5 and IR. Absolute quantification with standard curve in each plate. N=8 in each cohort and analyzed with two-tailed Student’s t-test using PRISM4 ***p<0.001. (b) To visualize localization, mice were treated with 100nM fluorescein labeled NPC86 for 48 hours. Mice were anesthetized and brains were collected. Following brains being submerged in 4% PFA solution and 30% sucrose, they were sectioned coronally at a thickness of 40 μm and imaged using Nikon’s A1R+ confocal microscope. IHC merged sagittal image for 100nM fluorescein NP-C86 and DAPI or NeuN. (c) Higher power image of dentate gyrus. Results indicate that NP-C86 crossed the blood-brain barrier and was taken up by the brain and was non-toxic to animals.
Desired Partnerships:
- License
- Sponsored Research
- Co-Development