Advantages:
- Compounds (peptidomimetic inhibitors) that prevent Doxorubicin-induced cardiomyopathy (DIC) that can occur with cancer chemotherapy
- Peptide therapeutics have high specificity, potency, and low toxicity when compared to traditional small molecule drugs
- Potentially enabling cancer patients to receive DOX treatment without compromising cardiac health
Summary:
Doxorubicin (DOX) is a widely used chemotherapy drug with severe side effects, including cardiomyopathy. DOX intercalates DNA leading to the death of cancer cells and normal cells. Preventing DOX-induced cardiomyopathy (DIC) is of urgent clinical need. Our researchers have identified the phosphatase complex PPP1R3G/PP1y, a key activator of receptor-interacting protein kinase 1 (RIPK1), which is involved in both apoptosis and necroptosis, two cell death pathways associated with cardiac diseases.
The researchers address the pressing clinical challenge of preventing Doxorubicin-induced cardiomyopathy (DIC) by targeting the phosphatase complex PPP1R3G/PP1y, a key activator of receptor-interacting protein kinase 1 (RIPK1). Successful in-vivo mouse models have shown promising results, with inhibiting PPP1R3G/PP1y leading to better-preserved cardiac function and increased survival rates following DOX treatment. They developed peptide-based inhibitors (peptidomimetics) that specifically block PPP1R3G/PP1γ interaction and thus inhibit RIPK1-dependent cell death. These are a new class of peptidomimetics called γ-AApeptides, which are derived from the chiral PNA backbone. The lead compound, pgb-1, repressed cell death in vitro and prevented mortality in TNF-induced SIRS. This technology holds promise in advancing cancer care and potentially enabling cancer patients to receive DOX treatment without compromising cardiac health.
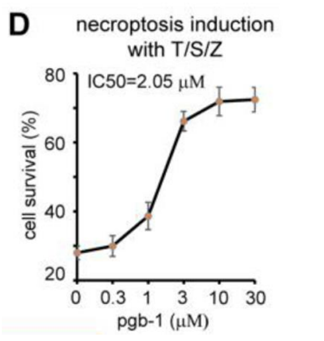
Top: Dose Response Curve of lead compound pgb-1 inhibiting necroptosis. (IC50 = 2uM)
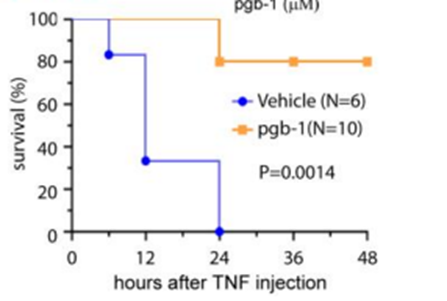
Bottom: Co-injection of pgb-1 protected 80% of mice from mortality caused by TNF-induced SIRS. WT mice received an intraperitoneal injection of either vehicle or 12 mg/kg pgb-1. 4hrs later, TNF was injected through tail vein and mortality was monitored.
Desired Partnerships: