Advantages
- By modulating E3 ligase activity, it is possible to regulate protein degradation pathways, gene activation, and other cellular functions.
- The research expands the therapeutic landscape by introducing the concept of E3 activators alongside E3 inhibitors, PROTACs, and molecular glues.
- Synthetic ligands, such as γ-AA peptides, offer versatility in ligand design and screening. This flexibility enables the development of ligands with desired properties for targeting specific E3 ligases or catalytic domains.
Summary
This research is focused on development of therapeutic interventions by leveraging the potential of E3 ligases. E3 ligases play essential roles in regulating protein degradation and are involved in various cellular processes, including protein homeostasis, gene activation, and cell cycle regulation. The research is centered around the manipulation of E6 associated protein (E6AP), an E3 ligase that is coerced by the human papillomavirus (HPV). E6AP is involved in the degradation of p53 protein, which allows HPV to invade host’s antiviral response and promote viral infection leading to tumorigenesis. This study aimed to identify activators of E6AP, specifically γ-AA peptides, that bind to the HECT domain of E6AP and stimulate ubiquitination and degradation of target proteins. Through affinity-based screening, they discovered that a γ-AA peptide called P6 showed significant affinity for the E6AP-HECT domain. P6 enhanced the ubiquitination and degradation of E6AP substrates, suggesting its potential as an E3 activator. It also suggests a new therapeutic approach based on the identification of E3-activating ligands, which could be used in combination with E3 inhibitors and substrate recruiters to manipulate protein degradation pathways for therapeutic purposes.
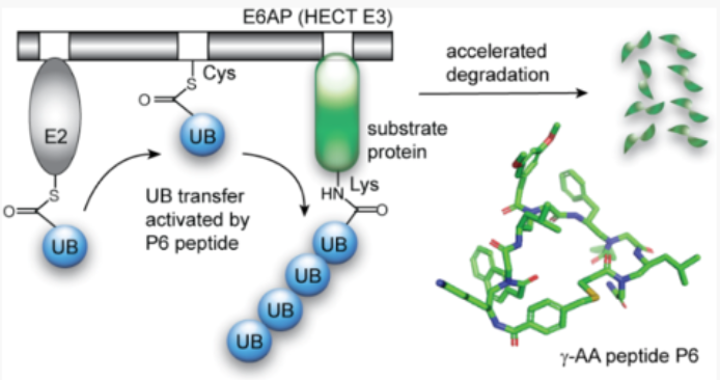
Ubiquitination and degradation of E6AP substrates using γ-AA peptide P6
Desired Partnerships
- License
- Sponsored Research
- Co-Development