Competitive Advantages
- Chemo diversity, enhanced conformational rigidity, and greater protease resistance
- Increased conformational constraint and binding affinity
- Proven method of drug discovery
Summary
Combinatorial chemistry serves as a powerful tool for ligand screening against potential protein and peptide targets. However, macrocyclic peptidomimetic combinatorial libraries are virtually nonexistent. Described is a new class of peptidomimetic macrocyclic combinatorial library with use as ligands for the Ephrin type-A receptor 2 (EphA2), which provides a novel method of drug discovery. The library is based on the use of peptide mimicking molecules with γ-AApeptide backbones and macrocyclic functional groups for binding specific biological targets. The advantages are the unique structure of its ligands as macrocyclic and peptidomimetic molecules. The γ-AApeptide backbones ensure chemo diversity, enhanced conformational rigidity, and greater protease resistance. This has been used successfully to identify a ligand targeting receptor tyrosine kinase, a protein involved in melanoma, ovarian, breast, and lung cancers, but has unlimited applications.
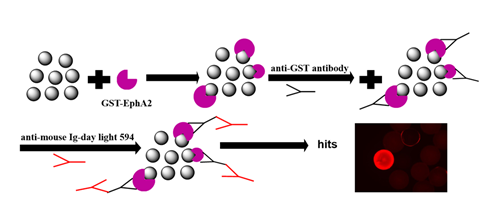
Screening of the γ-AApeptide Library
Desired Partnerships
- License
- Sponsored Research
- Co-Development