Advantages
- Peptidomimetics directly enhances PTEN's lipid phosphatase activity and reduces oncogenic PI3K/AKT signaling
- α-AApeptides allosterically modulate PTEN at specific binding interfaces, enhancing phosphatase activity while minimizing unintended cellular effects
- By improving PTEN function, α-AApeptides can complement current treatments such as standard chemotherapy or targeted kinase inhibitors
- α-AApeptides could provide broad utility wherever PTEN function is compromised, opening avenues for treating various cancers beyond lung adenocarcinoma
Summary
Tumor suppression through regulation of the PI3K/AKT signaling pathway is a critical target in cancer therapy, particularly as one of the key negative regulators is often mutated, lost, or dysregulated in many malignancies. Current strategies predominantly rely on inhibiting kinases in the pathway, but these approaches face several limitations, including off-target effects, drug resistance, and the inability to restore function of essential regulators whose expression or conformation may be compromised. Moreover, achieving precise control of this signaling cascade remains challenging because conventional therapies do not directly stabilize or activate the central phosphatase responsible for reversing aberrant pathway activation. As a result, new solutions are sought that can specifically modulate the tumor suppressor’s structure and catalytic activity, offering potentially safer and more durable interventions for various cancers.
This invention introduces α-AApeptide-based PTEN activators, which enhance PTEN’s lipid phosphatase activity by binding at the interface of its phosphatase and C2 domains and near the CBR3-loop, thereby restraining the PI3K/AKT/S6K pathway. Structurally, these peptidomimetics require an adamantyl substituent at R3, an aromatic side chain at R2, and a hydrophilic moiety at R1 to induce conformational changes that promote PTEN’s active state. In lung cancer cells, the lead compounds (#43 and #9) decrease phosphorylated AKT and P70S6K levels, reduce proliferation and migration, and trigger cell cycle arrest, showcasing their efficacy. Unlike traditional kinase inhibitors that often face drug resistance and off-target effects, these α-AApeptides directly target PTEN, providing a novel therapeutic strategy to restore tumor suppressor function.
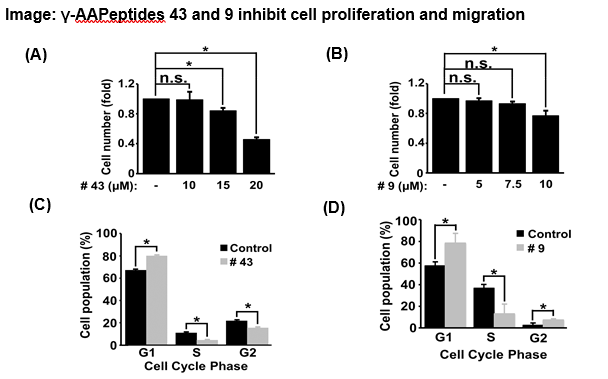
(A) Dose-dependent inhibition of A549 cell proliferation was observed in A549 cells treated for 24 hours with the indicated concentration of γ-AA Peptide #43. (B) Dose-dependent inhibition of A549 cell proliferation was observed in A549 cells treated for 24 hours with an indicated concentration of γ-AA Peptide #9. (C) Cell cycle analysis of A549 cells treated for 24 hours with 40μM γ-AA Peptide #43 shows an increase in G1 cell population with a decreased S phase and G2 phase cell population, n=3 (*p value ≤0.05). (D) Cell cycle analysis of A549 cells treated for 24 hours with 20μM γ-AA Peptide #9 shows an increase in the G1 cell population, a decreased S phase population, and an increase in the G2 phase cell population, n=3 (*p value ≤0.05).
Desired Partnership: