Competitive Advantages
- Reduces neural damage
- Not dependent on type of stroke
- Conivaptan is already FDA-approved
- Greater therapeutic window than current stroke medications
Summary
USF researchers have developed a method of treating a stroke or suspected stroke by administering a pharmaceutical formulation comprised of conivaptan within 6 hours of stroke occurrence followed by an additional administration within a prescribed time period. Preliminary results have shown that this treatment method may reduce neuronal infarct size, neuronal edema and/or neuronal inflammation in a subject following a stroke. This method of treatment using a FDA-approved agent holds great therapeutic potential in stroke treatment.
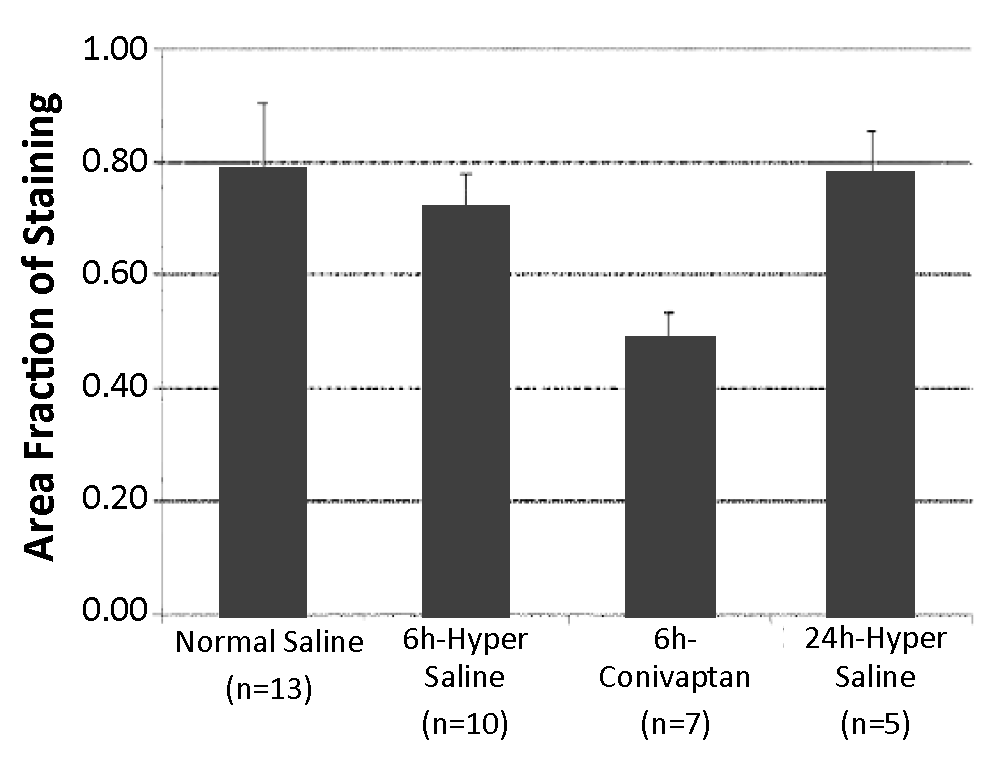
The Average Area Fraction of CD11b Staining at 48 Hours Post Stroke
Desired Partnership