Competitive Advantages
- A prognostic genomic-based method
- Improved survival prediction accuracies
- Improved immunotherapy effectiveness predictions
- Measures tumor immune activity
Summary
USF researchers have developed a genomics-based method to provide prognoses based on tumor immune activity. Also provided are indications for components for effective immunotherapy approaches to cancer treatment. Specifically, this method is able to determine whether tumor sample lymphocytes have T-cell receptor (TCR) complementarity-determining region 3 (CDR3) chemical features that correspond with the chemical features of a mutant tumor peptide in such a way as to indicate both better survival for the patient and possibly the effectiveness of various immunotherapy approaches. This bioinformatics assessment method of cancer-associated TCR biochemical features examines combinations of T-cell receptor CDR3 amino acid usage with human leukocyte antigen types. It then quantifies and evaluates cancer survival statistics. Finally, these CDR3-muntant peptide complementarity assessments can be done with tumor exome files, often already available for other reasons. And, the assessments can be applicable to B-cell receptor as well as T-cell receptor, CDR3-mutant peptide complementarities.
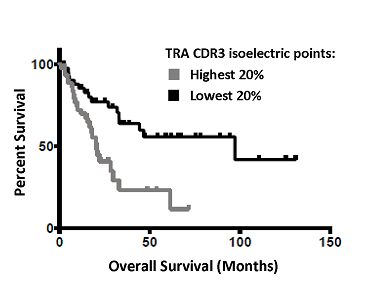
Survival Rates Associated with T-Cell Receptor Alpha CDR3 Isoelectric Points
Desired Partnerships
- License
- Sponsored Research
- Co-Development