Advantages
- Disrupts protein-protein interactions
- Mimics the α-helical domain
- More potent cellular activity
- Potential anti-cancer therapeutics
- Exhibits excellent folding stability with defined helices
- Demonstrates remarkable enzymatic stability for 24 hours
Summary
Researchers have developed sulfono-γ-AApeptides that are able to disrupt cancer-related protein-protein interactions (PPIs).
PPIs are imperative to life. Protein molecules interact due to biochemical events and can stimulate a series of occurrences such as cellular membrane transport, signal transduction, cellular metabolism processes and muscle contraction. PPIs are also vital to the survival of cancer cells and are therefore a good target for anti-cancer therapeutics. Researchers have attempted to modulate protein allosteric sites as well as other prevalent protein interaction areas for drug-design strategies. Unfortunately, the modulation of PPIs is very challenging and requires further development.
Our researchers have designed a series of helical peptides that disrupt necessary PPIs in B-cell acute lymphoblastic leukemia cancer cells. Our studies have shown that the novel peptides, termed sulfono-γ-AApeptides, can structurally and functionally mimic the secondary structure of B-cell lymphoma 9 (BCL9) proteins. They then selectively disrupt the PPIs between BCL9 proteins and β−catenin proteins. β−catenin proteins are known to play an important role in the cellular adhesion and gene transcription of cancer cells. This technology is able to enter cancer cells and disrupt their PPIs at a much greater potency than other available peptides. The design of these helical sulfono-γ-AApeptides could lead to the development of a new modulation strategy regarding PPIs. These peptides hold great potential in the development of anti-cancer therapeutics.
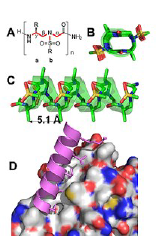
(A-C) Crystal structure of sulfono-γ-AApeptide; (D) Binding of a sulfono-γ-AApeptide to β−catenin and disrupt BCL9/ β−catenin PPI
Desired Partnerships
- License
- Sponsored Research
- Co-Development