Advantages
- Protects embedded molecules from degradation and toxicity by shielding them within the GFP β-barrel
- Enables simultaneous delivery of therapeutic/diagnostic agents and real-time imaging due to retained fluorescence
- Provides a versatile platform for embedding a wide range of non-native molecules (e.g., peptides, drugs, imaging agents)
- Facilitates targeted delivery of embedded agents to specific cells or organelles
- Enhances the stability and solubility of the chimeric protein through specific amino acid substitutions
Summary
The biotechnology landscape continuously seeks advanced strategies for delivering a diverse range of therapeutic, diagnostic, and prophylactic agents. Many promising biomolecules, including various peptides, possess significant potential for addressing critical health challenges. However, their inherent instability and susceptibility to degradation in biological environments often impede their clinical utility. Consequently, there is a pressing demand for innovative delivery platforms that can effectively shield these sensitive compounds, enhance their bioavailability, and facilitate their precise targeting to specific sites within the body.
This technology is distinct by utilizing GFP's inherent β-barrel as a unique, internal protective scaffold for sensitive molecules, unlike conventional methods that involve external fusion or encapsulation. This internal embedding offers superior protection against degradation and reduces toxicity during production, while critically maintaining GFP's fluorescent properties. The ability to simultaneously deliver diverse agents (e.g., therapeutics) and provide real-time imaging or tracking within a single, stable protein unit represents a significant advancement over existing delivery systems, which often lack integrated diagnostic capabilities or robust internal shielding.
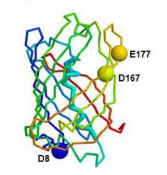
Optimized Structure of GFP-Bactenecin with Stabilizing Mutations
Desired Partnerships
- License
- Sponsored Research
- Co-Development